Company Overview
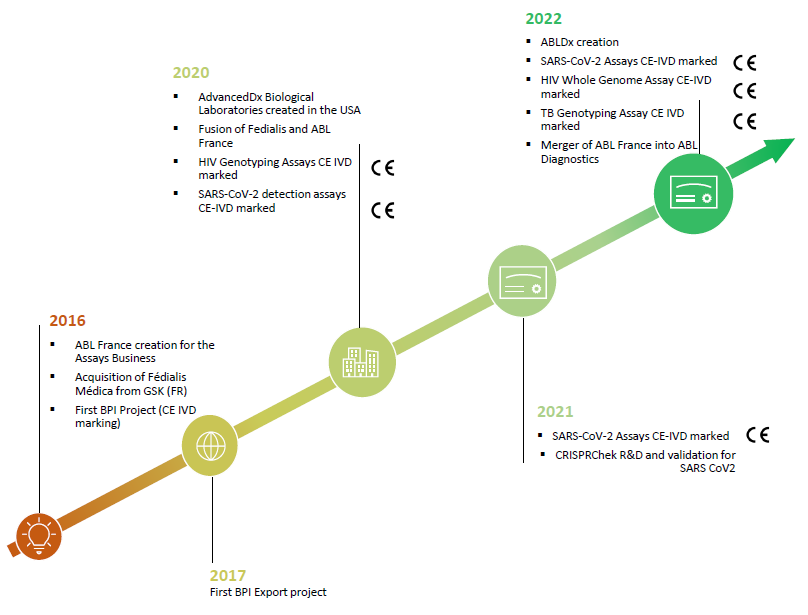
On September 1st 2022, Advanced Biological Laboratories Fedialis SAS (formerly ABL France SAS) merged into ABL Diagnostics S.A. (ABLD), a worldwide leading international company, listed on Euronext Paris compartment C (ISIN FR001400AHX6), offering innovative and proprietary molecular biology assays and end-to-end solutions intended to be used for molecular detection by Polymerase Chain Reaction (PCR) – UltraGene® and for genotyping through DNA sequencing – DeepChek® (a very sensitive, robust and sustainable technology allowing precise identification of relevant genomic variations like single nucleotide polymorphisms (SNP), amino-acid mutations, quasispecies like variants of concern, already published or which will be discovered in the future, with known impact on disease prognosis, drug efficacy, pathogen activity…).
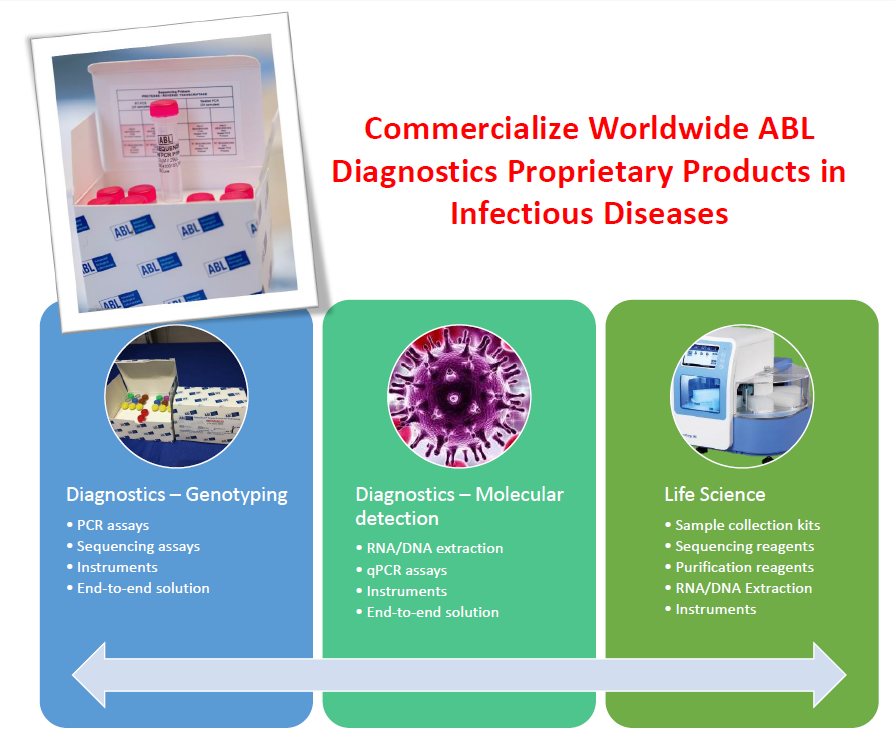
These molecular biology products are generating recurring revenues and cover one of the largest portfolio of microbiology applications, growing fast year after year to stick to the market needs, with a primary focus on HIV (with CE-IVD marked target-specific assays covering all relevant genes used for drug resistance assessment like reverse transcriptase, protease, integrase and with disruptive Whole Genome Kits), on SARS-CoV-2 (with a first version of the Whole Genome assay being CE-IVD), on Tuberculosis (with a CE-IVD marked multiplex assay targeting genes relevant for first line, second line and new-drugs resistance determination), on viral hepatitis B and C, 16s/18s RNA for taxonomy and microbiome analyses and other viral and bacterial targets. Please consult ABL team for further information about registration status of the ABLD’s products in your territory.
ABLD commercializes its entire line of products on a worldwide basis throught its own sales team and through a network of exclusive distributors active on all continents. ABLD clients are academic clinical pathology labs, private reference labs and researchers willing to implement an innovative and robust microbiology content in constant expansion.
ABLD also develops, manufactures and markets kits for clinical specimen collection – MediaChek® and digital solutions like Nadis®, an CE-marked Electronic Medical Record (EMR) system used in France in more than 200 hospitals managing patients infected by HIV or Viral Hepatitis.
Since 2019, the activities of ABL DIAGNOSTICS have also been carried out in the United States through its wholly owned subsidiary, ABL ADVANCEDDX BIOLOGICAL LABORATORIES USA Inc.